Kif/Yap 2021/2022 Project – The Effect of KIF11 Activity on YAP Localization
Luca Pizzale (Form VI), Elodie Wardle (Form VI), John Grissinger (Form V),
Matt Oatman (Form V), Katia Krishtopa (Form IV)
Project Summary + Project Goal
This project is founded upon targeted gene therapy and two potential proteins involved: KIF11 and YAP. KIF11, a motor protein, and YAP, a transcription factor are both potential drivers of cancer and possibly may be related to one another. Investigating these two drivers may provide helpful information in future targeted therapies which is why the group chose to focus specially on their unknown connection.
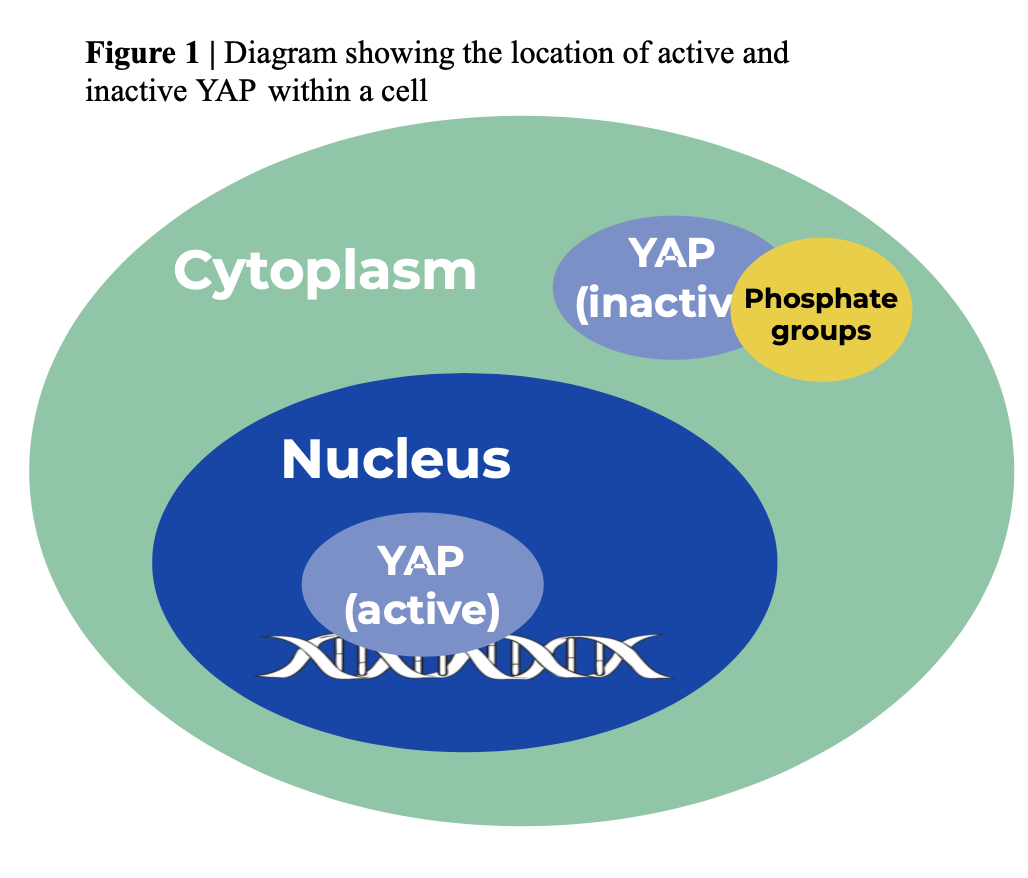
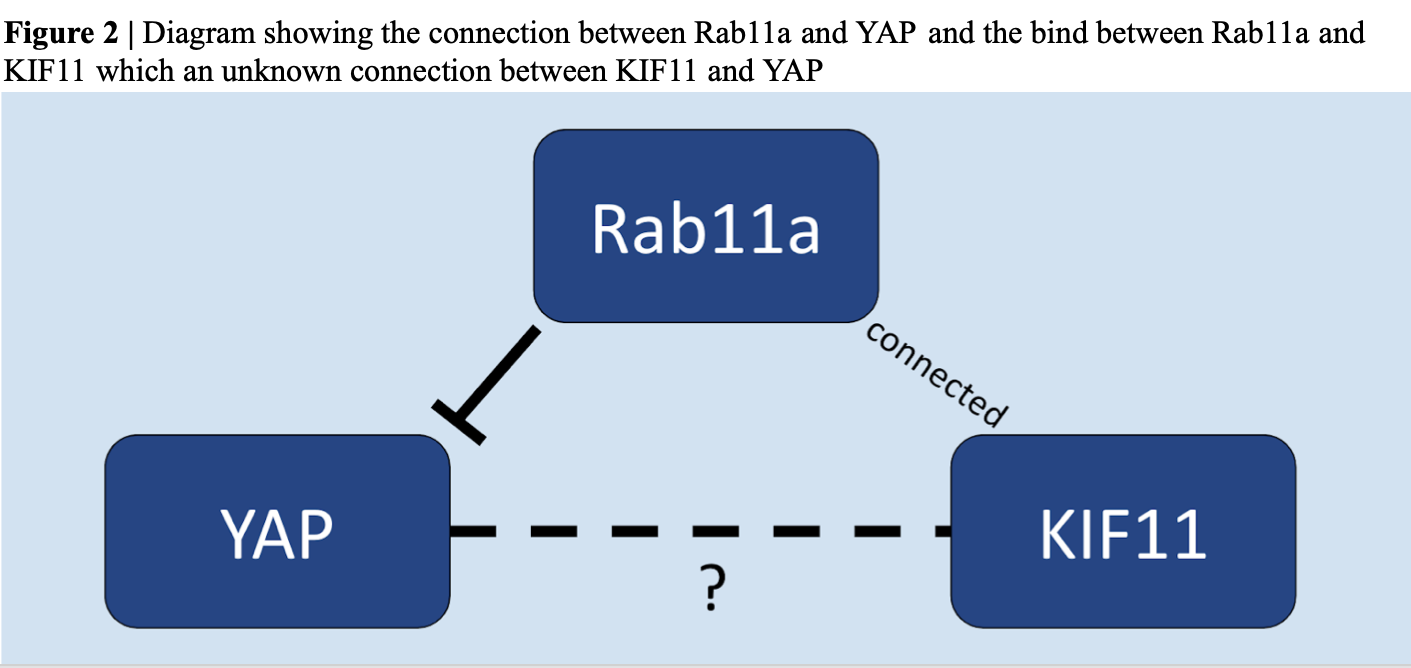
Upon consulting Dr. Gao at Rutgers Laboratory, the team hypothesized that KIF11 participates in the cellular transportation of YAP (in other words: KIF11 and YAP are connected). It was known that when active, YAP lives in the nucleus and when inactive, YAP is phosphorilated in the cytoplasm. From this past knowledge, the group developed a potential pathway to show KIF11 and YAP connections (Figure 1). Both protiens might be connected through Rab11a, a GTPase that regulates intercellular traffic. Rab11a has an inverse relationship with YAP (the less Rab11a leads to more activated YAP). Dr. Gao found that KIF11 was found to bind to Rab11a when intact which supported this pathway (Figure 2).
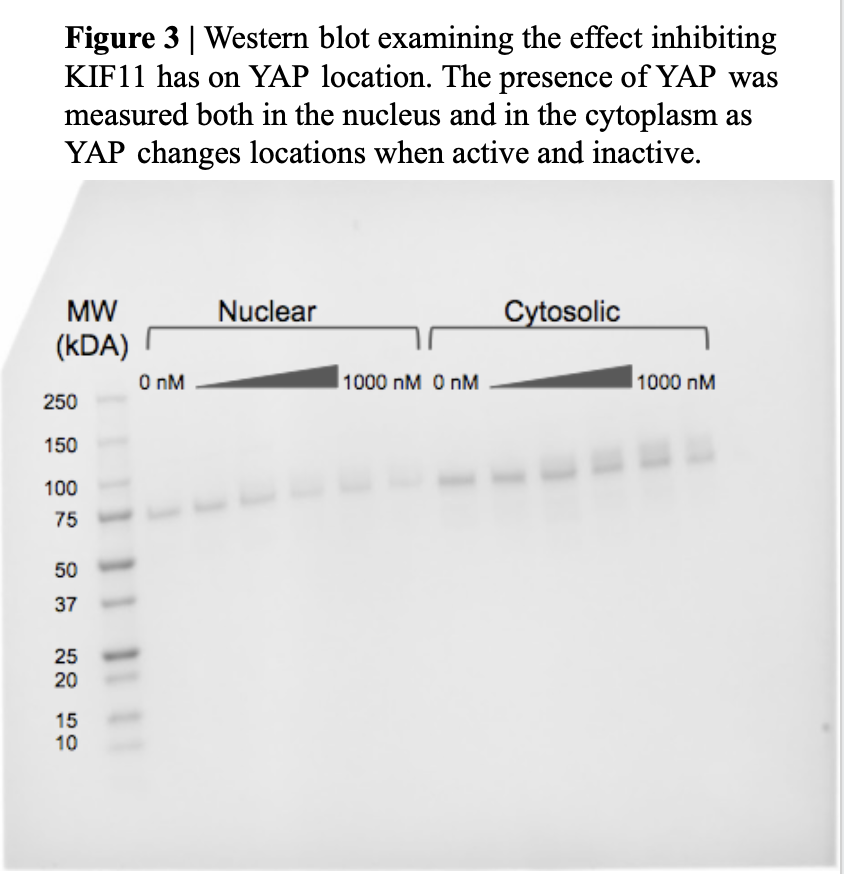
From our pathway, the group then focused on the question “If we inhibit KIF11 activity, does YAP’s location change”. The original hypothesis to answer this question is: as KIF11 is inhibited, YAP becomes inactive and moves to the cytoplasm. To do this, the group used SW480 cells (human colon cancer cells) provided by Dr. Gao, and grew, harvested, and froze them for experimentation. To inhibit KIF11, 6 plates were treated with ispinesib (concentrations ranging from 0 to 1000 nM) for 24 hours and then the contents of each plate were frozen and thawed to break down all cells. To separate the nuclear and cytosolic fragments of each cell, a centrifuge was used and then the cell contents were ran through a western blot test (Figure 3).
Current State
Our team has ran our final western blot to explore and confirm our hypothesis (Figure 3). This blot has shown us the presence of KIF11 and YAP as well as how the inhibition of KIF11 appears to change the location of YAP from the nucleus to the cytoplasm. The blot showed that as the concentration of Ispinesib increases, the protein band representing the nuclear YAP fades, indicating that the amount of YAP in the nucleus is decreasing. As it is known that YAP is in the nucleus when it is dephosphorylated and active, but it is in the cytoplasm when it is phosphorylated and inactive and the western suggests that KIF11 inhibition casques YAP to become phosphorylated and exit the nucleus, therefore becoming inactive. These results support the hypothesis that there is a connection between the proteins. Although the group had some staining difficulties on the last western blot (where the same proceidgers were followed) (Figure 4), what was visible coordinated directly with our previous blot which supported our past findings. Both blots have answered how KIF11 and YAP are connected as well as the location of both proteins when one is inhibited or activated. Due to time constraints, the group could not repeat our experiment once more or change the control variable to test accuracy, however the group is confident in our findings.
As we have proved our hypothesis, our project has concluded and will no longer be pursued in the upcoming years.
Next Steps
Our final goal is to share our findings though the Pingry Community Research Journal as we have concluded our project. We hope to publish the connection we made between KIF11 and YAP so that further research may be done on both proteins in the future.
Research Week Poster
References
Purcell, J. W. et al. (2010). Activity of the kinesin spindle protein inhibitor ispinesib (SB-715992) in models of breast cancer. Clinical Cancer Research, 16(2), 566-576.
D’Agostino, L. (2019). Recycling endosomes in mature epithelia restrain tumorigenic signaling. Cancer Research, 79(16), 4099-4112.
Meet the Team





Luca Pizzale
Elodie Wardle
John Grissinger
Matt Oatman
Katia Krishtopa

