The Effect of KIF11 Activity on YAP Localization
Lily Arrom, Luca Pizzale, Elodie Wardle, Dr. Sparrow
Project Summary
Our project is focused on the relationship between two proteins, KIF11 and YAP. KIF11 is a kinesin, otherwise known as the “tractor of the cell”, which is responsible for pulling chromosomes apart during mitosis. YAP, on the other hand, is a transcription factor that activates cell growth and sometimes plays a role in cancer. Inactive YAP has phosphate groups and is located in the cytoplasm, but when the Hippo pathway dephosphorylates YAP, it moves into the nucleus and becomes active (Figure 1). We’ve been in touch with Dr. Gao and his lab at Rutgers University, where they hypothesize that KIF11 and YAP are linked together via the interactions of other proteins (Figure 2). Our goal is to test this hypothesis by culturing SW480 cells, manipulating their expression of KIF11, and performing western blots to observe changes in YAP activation. Finding a new way to potentially manipulate YAP could prove helpful in targeted cancer therapies.
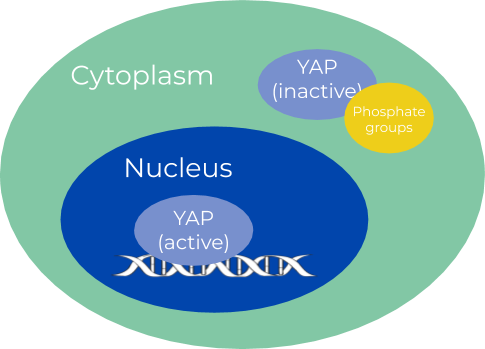
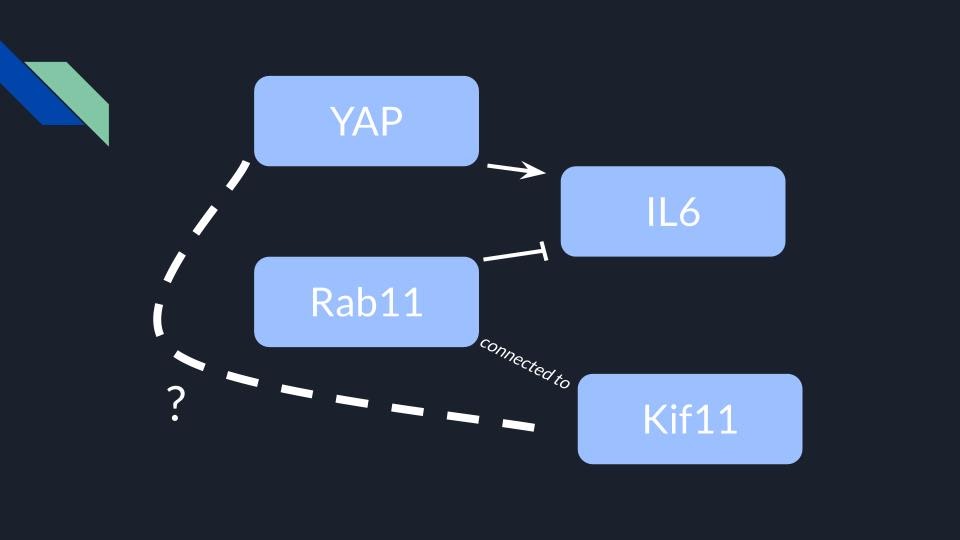
Figure 1: YAP’s location in the cell changes with its activation
Figure 2: YAP upregulates an interleukin named IL-6, which is inhibited by another protein named Rab11. Dr. Gao has observed that KIF11 is attached to Rab11, and we are collaborating with him to see if KIF11 and YAP have a more direct relationship.
Western Blot
The western blot is a staining method that shows the quantity of a specific protein in a varied sample, and it is crucial to our understanding of YAP’s localization. This process involves separating proteins by size, and then identifying the desired protein by introducing its primary antibody counterpart. This primary antibody is then paired with a secondary antibody, whose enzyme component can catalyze the conversion of a colorless substrate to create visible banding (visualized in Figure 3). If the resulting band is very dark, that means that there is a lot of the desired protein in the sample. You’ll also see that our blots have one or two lanes with multiple bands. These lanes were loaded with a molecular weight ladder, which acts as a benchmark for certain molecular weights (ex. we know that any protein in line with the top band would be 250 kDa).
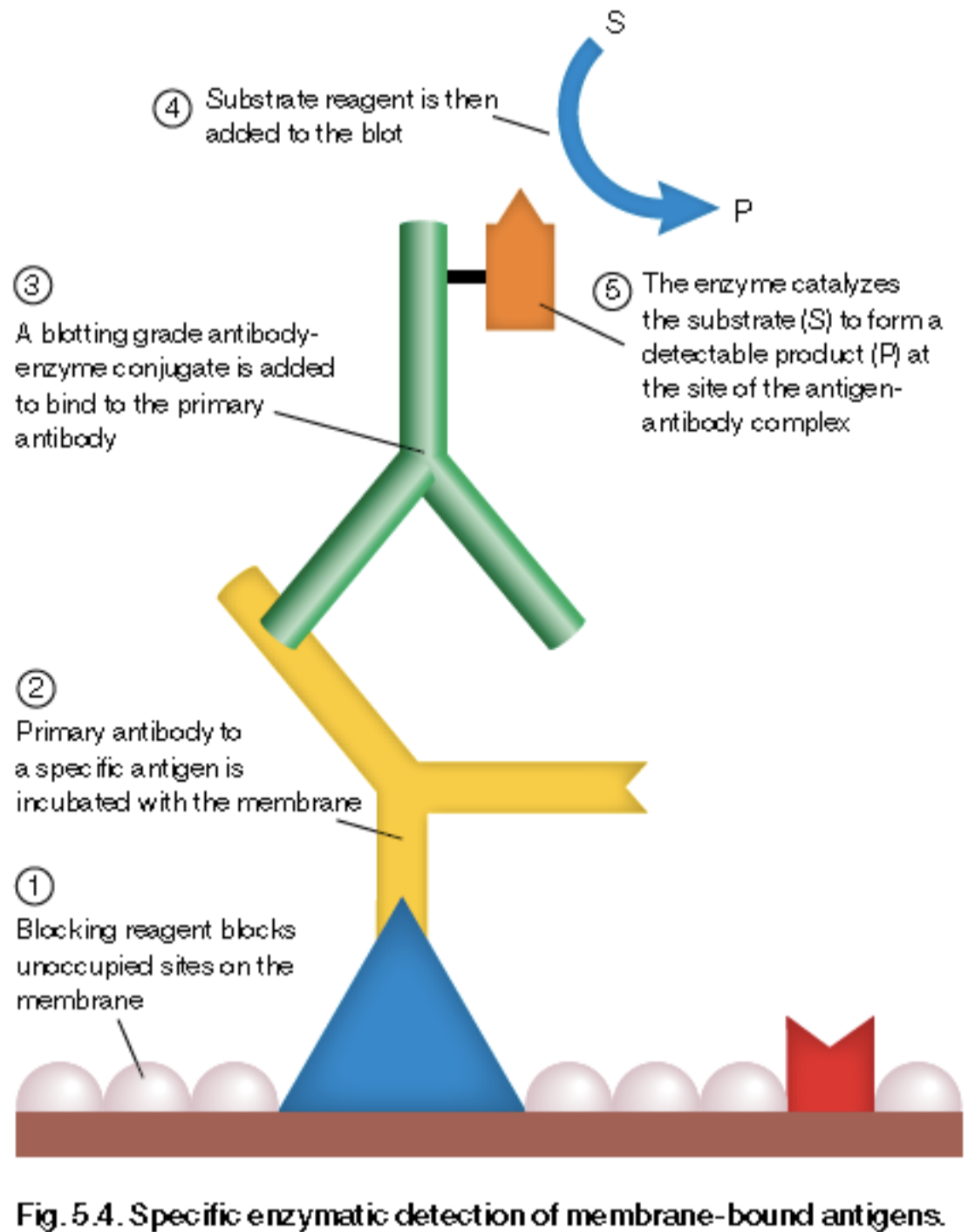
Figure 3: How a specific protein is identified in a western blot
Current State
During the 2018-19 school year, we obtained SW480 cells from Dr. Gao’s lab and began to culture them. These are human colon cancer cells that, when properly maintained, are capable of multiplying indefinitely and are therefore very useful to our study. Once we had cultured enough cells, we stored some away for future use and used the rest in our first western blot (Figure 4). This western was done to familiarize ourselves with the procedure, but also to confirm the presence of YAP and KIF11 in our cell samples.
In the 2019-2020 school year, our main goal was to reculture our cells, inhibit KIF11, and then perform a second western blot (Figure 5). Prior to beginning the western blot, we titrated the cells with varying concentrations of a drug called Ispinesib in order to inhibit KIF11. And, considering that YAP’s localization changes with its activation (Figure 1), we decided to split the cell’s nuclear components from the cytosol via centrifugation. We observed that, as the concentration of Ispinesib increases, nuclear YAP decreases, suggesting that the inhibition of KIF11 prevents YAP from being activated.
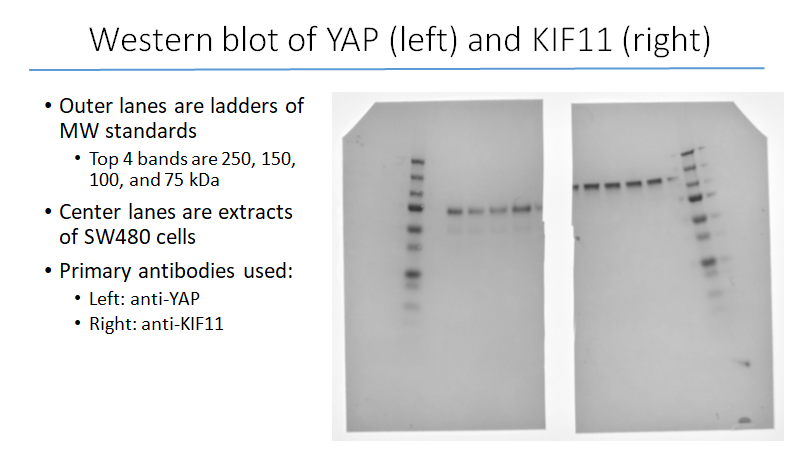
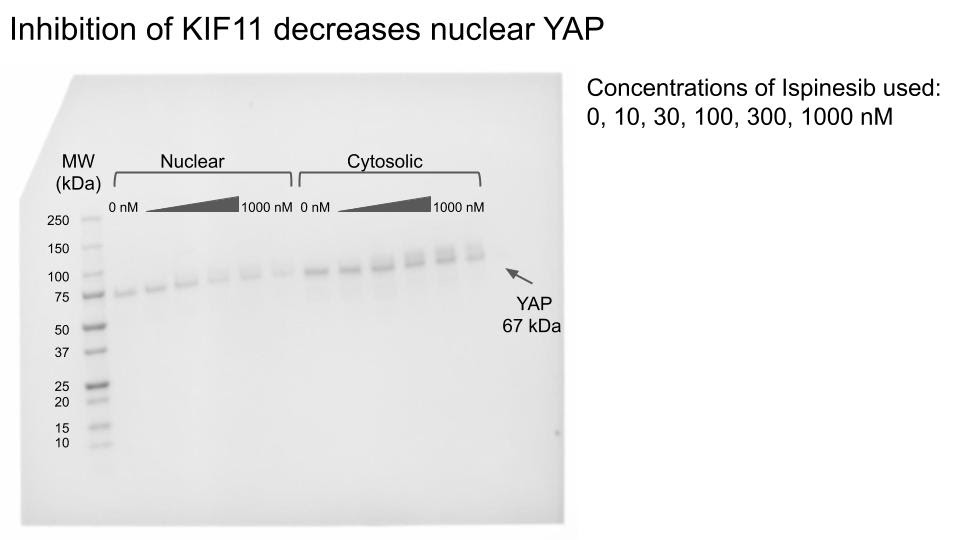
Figure 4: Western blot, May 2019
Figure 5: February 2020. Unlike the previous blot, this one was only stained for YAP.
Note how nuclear (active) YAP fades away as the Ispinesib concentration increases
Next Steps
Although our most recent western blot suggests that inactive KIF11 results in inactive YAP, we cannot confidently make that assertion based on only one experiment. Going forward we would like to repeat this experiment to see if we can reproduce the same results. And, after communicating with Dr. Gao about what we observed, he suggested that we also apply two other controls. The first would be to re-probe a blot with anti-histone antibodies. Histones are proteins only found in the nucleus, so staining for them would tell us if we succeeded in separating the nucleus from the cytosol. Second, we would also want to probe a future blot with an antibody exclusive to phosphorylated YAP. This would let us know if manipulating KIF11 impacts the Hippo pathway’s dephosphorylation of YAP. All of these future experiments would be completed with a fresh batch of SW480 cells, which we will be thawing and reculturing upon our return to campus.
Meet the Team




